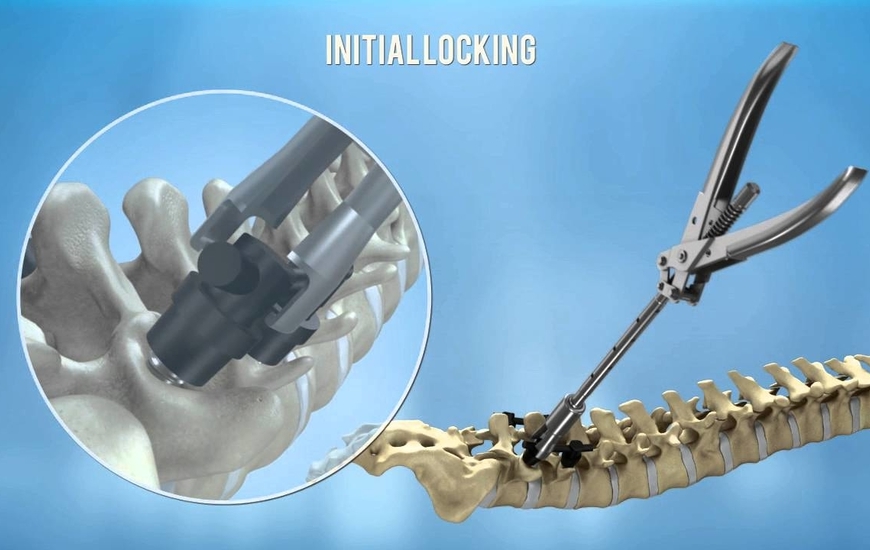
CarboFix has announced that the U.S. Food and Drug Administration (FDA) has cleared its CarboClear® Carbon Fiber Vertebral Body Replacement (VBR) System to replace a collapsed, damaged, or unstable vertebral body due to tumor or trauma.
The CarboClear® VBR System joins the FDA cleared CarboClear® Pedicle Screw and CarboClear® Fenestrated Pedicle Screw Systems.
The CarboClear® VBR implant is made of carbon fibers, with integrated porous Ti-alloy endplates.
Carbon fiber implants are proposing unique advantages to the oncological patients and their physicians. Among those advantages are: enhanced radiation therapy planning abilities, allowing radiation treatment to optimize the radiation dose to the tumor with minimal collateral tissue damage, and enhanced follow up abilities due to artifacts-free imaging.
-

-
18 October 2019